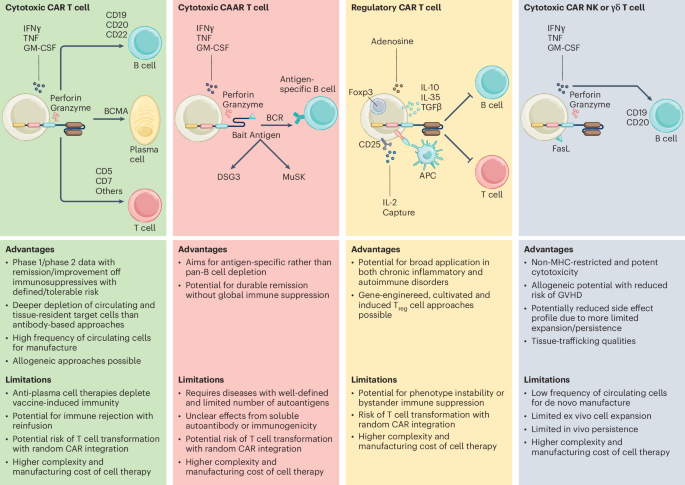
Clinical progress of engineered cellular immunotherapies for autoimmunity – Nature Biotechnology
Davenport, A. J. et al. CAR-T cells inflict sequential killing of multiple tumor target cells. Cancer Immunol. Res. 3, 483–494 (2015).
Google Scholar
Kalos, M. et al. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci. Transl. Med. 3, 95ra73 (2011).
Google Scholar
Maude, S. L. et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N. Engl. J. Med. 378, 439–448 (2018).
Google Scholar
Locke, F. L. et al. Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): a single-arm, multicentre, phase 1–2 trial. Lancet Oncol. 20, 31–42 (2019).
Google Scholar
Wang, M. et al. KTE-X19 CAR T-cell therapy in relapsed or refractory mantle-cell lymphoma. N. Engl. J. Med. 382, 1331–1342 (2020).
Google Scholar
Abramson, J. S. et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): a multicentre seamless design study. Lancet 396, 839–852 (2020).
Google Scholar
Roddie, C. et al. Obecabtagene autoleucel in adults with B-cell acute lymphoblastic leukemia. N. Engl. J. Med. 391, 2219–2230 (2024).
Google Scholar
Munshi, N. C. et al. Idecabtagene vicleucel in relapsed and refractory multiple myeloma. N. Engl. J. Med. 384, 705–716 (2021).
Google Scholar
San-Miguel, J. et al. Cilta-cel or standard care in lenalidomide-refractory multiple myeloma. N. Engl. J. Med. 389, 335–347 (2023).
Google Scholar
Sadelain, M. CAR therapy: the CD19 paradigm. J. Clin. Invest. 125, 3392–3400 (2015).
Google Scholar
Cappell, K. M. & Kochenderfer, J. N. Long-term outcomes following CAR T cell therapy: what we know so far. Nat. Rev. Clin. Oncol. 20, 359–371 (2023).
Google Scholar
Abeles, I. et al. B cell-directed therapy in autoimmunity. Annu. Rev. Immunol. 42, 103–126 (2024).
Google Scholar
Vital, E. M. et al. B cell biomarkers of rituximab responses in systemic lupus erythematosus. Arthritis Rheum. 63, 3038–3047 (2011).
Google Scholar
Anolik, J. H. et al. Delayed memory B cell recovery in peripheral blood and lymphoid tissue in systemic lupus erythematosus after B cell depletion therapy. Arthritis Rheum. 56, 3044–3056 (2007).
Google Scholar
Reddy, V. R. et al. Disparity in peripheral and renal B-cell depletion with rituximab in systemic lupus erythematosus: an opportunity for obinutuzumab? Rheumatology 61, 2894–2904 (2022).
Google Scholar
Mouquet, H. et al. B-cell depletion immunotherapy in pemphigus: effects on cellular and humoral immune responses. J. Invest. Dermatol. 128, 2859–2869 (2008).
Google Scholar
Hammers, C. M. et al. Persistence of anti-desmoglein 3 IgG+ B-cell clones in pemphigus patients over years. J. Invest. Dermatol. 135, 742–749 (2015).
Google Scholar
Kavanaugh, A. et al. Assessment of rituximab’s immunomodulatory synovial effects (ARISE trial). 1: clinical and synovial biomarker results. Ann. Rheum. Dis. 67, 402–408 (2008).
Google Scholar
Yeung, C. C. S. et al. Abnormal bone marrow findings in patients following treatment with chimeric antigen receptor-T cell therapy. Eur. J. Haematol. 112, 111–121 (2024).
Google Scholar
O’Reilly, M. et al. Trafficking of CAR T cells to sites of subclinical leukaemia cutis. Lancet Oncol. 21, e179 (2020).
Google Scholar
Siddiqi, T. et al. CD19-directed CAR T-cell therapy for treatment of primary CNS lymphoma. Blood Adv. 5, 4059–4063 (2021).
Google Scholar
Kansal, R. et al. Sustained B cell depletion by CD19-targeted CAR T cells is a highly effective treatment for murine lupus. Sci. Transl. Med. 11, eaav1648 (2019).
Google Scholar
Jin, X. et al. Therapeutic efficacy of anti-CD19 CAR-T cells in a mouse model of systemic lupus erythematosus. Cell. Mol. Immunol. 18, 1896–1903 (2021).
Google Scholar
Mougiakakos, D. et al. CD19-targeted CAR T cells in refractory systemic lupus erythematosus. N. Engl. J. Med. 385, 567–569 (2021).
Google Scholar
Muller, F. et al. CD19 CAR T-cell therapy in autoimmune disease—a case series with follow-up. N. Engl. J. Med. 390, 687–700 (2024). This is the largest case series of anti-CD19 CAR T cells in autoimmunity published to date, involving eight individuals with SLE who all achieved DORIS remission, three individuals with IIM who achieved ACR-EULAR major clinical response and four individuals with SSc who demonstrated improved EUSTAR activity index. All 15 individuals discontinued immunosuppressive therapy. The mean period of B cell depletion was 112 days. Adverse events included grade 1 CRS (n = 10), grade 2 CRS (n = 1), grade 1 ICANS (n = 1) and pneumonia resulting in hospitalization (n = 1).
Google Scholar
Mackensen, A. et al. Anti-CD19 CAR T cell therapy for refractory systemic lupus erythematosus. Nat. Med. 28, 2124–2132 (2022).
Google Scholar
Nordmann-Gomes, A. et al. CAR T-cell therapy in SLE: a systematic review. Semin. Arthritis Rheum. 74, 152786 (2025).
Google Scholar
Bergmann, C. et al. Treatment of a patient with severe systemic sclerosis (SSc) using CD19-targeted CAR T cells. Ann. Rheum. Dis. 82, 1117–1120 (2023).
Google Scholar
Auth, J. et al. CD19-targeting CAR T-cell therapy in patients with diffuse systemic sclerosis: a case series. Lancet Rheumatol. 7, e83–e93 (2025).
Google Scholar
Pecher, A. C. et al. CD19-targeting CAR T cells for myositis and interstitial lung disease associated with antisynthetase syndrome. JAMA 329, 2154–2162 (2023).
Google Scholar
Merkt, W. et al. Third-generation CD19.CAR-T cell-containing combination therapy in Scl70+ systemic sclerosis. Ann. Rheum. Dis. 83, 543–546 (2024).
Google Scholar
Wang, X. et al. Allogeneic CD19-targeted CAR-T therapy in patients with severe myositis and systemic sclerosis. Cell 187, 4890–4904 (2024).
Google Scholar
Muller, F. et al. CD19-targeted CAR T cells in refractory antisynthetase syndrome. Lancet 401, 815–818 (2023).
Google Scholar
Nicolai, R. et al. Autologous CD19-targeting CAR T cells in a patient with refractory juvenile dermatomyositis. Arthritis Rheumatol. 76, 1560–1565 (2024).
Google Scholar
Taubmann, J. et al. Rescue therapy of antisynthetase syndrome with CD19-targeted CAR-T cells after failure of several B-cell depleting antibodies. Rheumatology 63, e12–e14 (2024).
Google Scholar
Volkov, J. et al. Case study of CD19 CAR T therapy in a subject with immune-mediate necrotizing myopathy treated in the RESET-Myositis phase I/II trial. Mol. Ther. 32, 3821–3828 (2024).
Google Scholar
Haghikia, A. et al. Clinical efficacy and autoantibody seroconversion with CD19-CAR T cell therapy in a patient with rheumatoid arthritis and coexisting myasthenia gravis. Ann. Rheum. Dis. 83, 1597–1598 (2024).
Google Scholar
Lidar, M. et al. CD-19 CAR-T cells for polyrefractory rheumatoid arthritis. Ann. Rheum. Dis. 84, 370–372 (2025).
Google Scholar
Szabo, D. et al. Sustained drug-free remission in rheumatoid arthritis associated with diffuse large B-cell lymphoma following tandem CD20–CD19-directed non-cryopreserved CAR-T cell therapy using zamtocabtagene autoleucel. RMD Open 10, e004727 (2024).
Google Scholar
Li, Y. et al. Fourth-generation chimeric antigen receptor T-cell therapy is tolerable and efficacious in treatment-resistant rheumatoid arthritis. Cell Res. 35, 220–223 (2025).
Google Scholar
Haghikia, A. et al. Anti-CD19 CAR T cells for refractory myasthenia gravis. Lancet Neurol. 22, 1104–1105 (2023).
Google Scholar
Motte, J. et al. Treatment of concomitant myasthenia gravis and Lambert–Eaton myasthenic syndrome with autologous CD19-targeted CAR T cells. Neuron 112, 1757–1763 (2024).
Google Scholar
Muppidi, S. et al. Utilization of MG-ADL in myasthenia gravis clinical research and care. Muscle Nerve 65, 630–639 (2022).
Google Scholar
Fischbach, F. et al. CD19-targeted chimeric antigen receptor T cell therapy in two patients with multiple sclerosis. Med 5, 550–558 (2024).
Google Scholar
Richter, J. et al. CD19-directed CAR T cell therapy in 4 patients with refractory multiple sclerosis. Blood 144, 2073–2073 (2024).
Google Scholar
ACTRIMS Forum 2025—Posters. Mult. Scler. J. 31, 24–244 (2025).
Minopoulou, I. et al. Anti-CD19 CAR T cell therapy induces antibody seroconversion and complete B cell depletion in the bone marrow of a therapy-refractory patient with ANCA-associated vasculitis. Ann. Rheum. Dis. 84, e4–e7 (2025).
Google Scholar
Trautmann-Grill, K. et al. Salvage treatment of multi-refractory primary immune thrombocytopenia with CD19 CAR T cells. Lancet 405, 25–28 (2025).
Google Scholar
Schultze-Florey, C. R. et al. Anti-CD19 CAR-T cell therapy for acquired hemophilia A. Leukemia 39, 980–982 (2025).
Google Scholar
Brudno, J. N. & Kochenderfer, J. N. Current understanding and management of CAR T cell-associated toxicities. Nat. Rev. Clin. Oncol. 21, 501–521 (2024).
Google Scholar
Shu, J. et al. Safety and clinical efficacy of relmacabtagene autoleucel (relma-cel) for systemic lupus erythematosus: a phase 1 open-label clinical trial. EClinicalMedicine 83, 103229 (2025).
Google Scholar
Cappell, K. M. & Kochenderfer, J. N. A comparison of chimeric antigen receptors containing CD28 versus 4-1BB costimulatory domains. Nat. Rev. Clin. Oncol. 18, 715–727 (2021).
Google Scholar
Feucht, J. & Sadelain, M. Function and evolution of the prototypic CD28ζ and 4-1BBζ chimeric antigen receptors. Immunooncol. Technol. 8, 2–11 (2020).
Google Scholar
Hagen, M. et al. Local immune effector cell-associated toxicity syndrome in CAR T-cell treated patients with autoimmune disease: an observational study. Lancet Rheumatol. 7, e424–e433 (2025).
Google Scholar
Bhoj, V. G. et al. Persistence of long-lived plasma cells and humoral immunity in individuals responding to CD19-directed CAR T-cell therapy. Blood 128, 360–370 (2016).
Google Scholar
Verdun, N. & Marks, P. Secondary cancers after chimeric antigen receptor T-cell therapy. N. Engl. J. Med. 390, 584–586 (2024).
Google Scholar
Storgard, R., Rejeski, K., Perales, M. A., Goldman, A. & Shouval, R. T-cell malignant neoplasms after chimeric antigen receptor T-cell therapy. JAMA Oncol. 10, 826–828 (2024).
Google Scholar
Harrison, S. J. et al. CAR+ T-cell lymphoma after cilta-cel therapy for relapsed or refractory myeloma. N. Engl. J. Med. 392, 677–685 (2025).
Google Scholar
Dulery, R. et al. T cell malignancies after CAR T cell therapy in the DESCAR-T registry. Nat. Med. 31, 1130–1133 (2025).
Google Scholar
Hamilton, M. P. et al. Risk of second tumors and T-cell lymphoma after CAR T-cell therapy. N. Engl. J. Med. 390, 2047–2060 (2024).
Google Scholar
Ozdemirli, M. et al. Indolent CD4+ CAR T-cell lymphoma after cilta-cel CAR T-cell therapy. N. Engl. J. Med. 390, 2074–2082 (2024).
Google Scholar
Braun, T. et al. Multiomic profiling of T cell lymphoma after therapy with anti-BCMA CAR T cells and GPRC5D-directed bispecific antibody. Nat. Med. 31, 1145–1153 (2025).
Google Scholar
Qin, C. et al. Anti-BCMA CAR T-cell therapy CT103A in relapsed or refractory AQP4-IgG seropositive neuromyelitis optica spectrum disorders: phase 1 trial interim results. Signal Transduct. Target. Ther. 8, 5 (2023). The largest case series of anti-BCMA CAR T cells in autoimmunity (NMOSD) showed that 11 of 12 patients achieved drug-free CR and 10 of 12 patients also achieved serologic remission. Hypogammaglobulinemia occurred in all patients who reached 6-month follow-up, associated with infectious SEAs.
Google Scholar
Muller, F. et al. BCMA CAR T cells in a patient with relapsing idiopathic inflammatory myositis after initial and repeat therapy with CD19 CAR T cells. Nat. Med. 31, 1793–1797 (2025).
Google Scholar
Qin, C. et al. Anti-BCMA CAR-T therapy in patients with progressive multiple sclerosis. Cell 188, P6414–P6423 (2025).
Google Scholar
Tipton, C. M. et al. Diversity, cellular origin and autoreactivity of antibody-secreting cell population expansions in acute systemic lupus erythematosus. Nat. Immunol. 16, 755–765 (2015).
Google Scholar
Wang, W. et al. BCMA-CD19 compound CAR T cells for systemic lupus erythematosus: a phase 1 open-label clinical trial. Ann. Rheum. Dis. 83, 1304–1314 (2024). This is the largest published case series of dual CD19–BCMA-targeting CAR T cells in autoimmunity to date. Nine of 13 individuals with SLE achieved CR within 6 months; in 12 of 13, anti-dsDNA and other autoantibodies became undetectable. Hypogammaglobulinemia occurred in 100% of individuals, associated with one case of mild urinary tract infection and three cases of severe coronavirus disease-related pneumonia. Revaccination of one individual after CART therapy resulted in restoration of protective anti-hepatitis B titers.
Google Scholar
Shen, N. et al. Clinical impact of C-CAR168, a novel anti-CD20/BCMA composite autologous CAR T therapy, in refractory lupus nephritis. J. Rheumatol. 52, 25–26 (2025).
Google Scholar
Wong, D. P. et al. A BAFF ligand-based CAR-T cell targeting three receptors and multiple B cell cancers. Nat. Commun. 13, 217 (2022).
Google Scholar
Luo, Y. et al. Translational development of a novel BAFF-R CAR-T therapy targeting B-cell lymphoid malignancies. Cancer Immunol. Immunother. 72, 4031–4047 (2023).
Google Scholar
Granit, V. et al. Safety and clinical activity of autologous RNA chimeric antigen receptor T-cell therapy in myasthenia gravis (MG-001): a prospective, multicentre, open-label, non-randomised phase 1b/2a study. Lancet Neurol. 22, 578–590 (2023).
Google Scholar
Chahin, N. et al. Durability of response to B-cell maturation antigen-directed mRNA cell therapy in myasthenia gravis. Ann. Clin. Transl. Neurol. 12, 2358–2366 (2025).
Google Scholar
Ellebrecht, C. T. et al. Reengineering chimeric antigen receptor T cells for targeted therapy of autoimmune disease. Science 353, 179–184 (2016).
Google Scholar
Lee, J. et al. Antigen-specific B cell depletion for precision therapy of mucosal pemphigus vulgaris. J. Clin. Invest. 130, 6317–6324 (2020). This paper highlights the preclinical data leading to FDA clearance of the DSG3-CAART Investigational New Drug application, representing the first highly targeted precision cellular immunotherapy to enter clinical trials for an autoimmune disease indication.
Google Scholar
Oh, S. et al. Precision targeting of autoantigen-specific B cells in muscle-specific tyrosine kinase myasthenia gravis with chimeric autoantibody receptor T cells. Nat. Biotechnol. 41, 1229–1238 (2023).
Google Scholar
Oh, S., Khani-Habibabadi, F., O’Connor, K. C. & Payne, A. S. Composition and function of AChR chimeric autoantibody receptor T cells for antigen-specific B cell depletion in myasthenia gravis. Sci. Adv. 11, eadt0795 (2025).
Google Scholar
Reincke, S. M. et al. Chimeric autoantibody receptor T cells deplete NMDA receptor-specific B cells. Cell 186, 5084–5097 (2023).
Google Scholar
Seifert, L. et al. An antigen-specific chimeric autoantibody receptor (CAAR) NK cell strategy for the elimination of anti-PLA2R1 and anti-THSD7A antibody-secreting cells. Kidney Int. 105, 886–889 (2024).
Google Scholar
Altun B, et al. Preclinical feasibility of antigen-specific B-cell depletion for phospholipase A2 receptor membranous nephropathy with chimeric autoantibody receptor T-cells. Kidney Intl. 109, 89–100 (2026).
Meng, H. et al. La/SSB chimeric autoantibody receptor modified NK92MI cells for targeted therapy of autoimmune disease. Clin. Immunol. 192, 40–49 (2018).
Google Scholar
Zhou, J. et al. GPIbα CAAR T cells function like a Trojan horse to eliminate autoreactive B cells to treat immune thrombocytopenia. Haematologica 109, 2256–2270 (2024).
Google Scholar
Peng, J. J. et al. Chimeric autoantibody receptor T cells clonally eliminate B cells producing autoantibodies against IFN-γ. Sci. Immunol. 10, eadm8186 (2025).
Google Scholar
Payne, A. S. et al. Clinical and translational data from a first-in-human study of a novel precision cellular immunotherapy (DSG3-CAART) in mucosal pemphigus vulgaris. J. Invest. Dermatol. 145, S75 (2025).
Google Scholar
Funakoshi, T. et al. Enrichment of total serum IgG4 in patients with pemphigus. Br. J. Dermatol. 167, 1245–1253 (2012).
Google Scholar
Volkov, J.R. et al. Clinical and translational findings following MuSK-CAART infusion without preconditioning in patients with myasthenia gravis (MuSCAARTes trial). Hum. Gene Ther. 36, P0744 (2025).
Maciocia, N., Wade, B. & Maciocia, P. CAR T-cell therapies for T-cell malignancies: does cellular immunotherapy represent the best chance of cure? Blood Adv. 9, 913–923 (2025).
Google Scholar
Angelos, M. G., Patel, R. P., Ruella, M. & Barta, S. K. Progress and pitfalls of chimeric antigen receptor T cell immunotherapy against T cell malignancies. Transplant. Cell. Ther. 30, 171–186 (2024).
Google Scholar
Liu, J. et al. Targeted CD7 CAR T-cells for treatment of T-lymphocyte leukemia and lymphoma and acute myeloid leukemia: recent advances. Front. Immunol. 14, 1170968 (2023).
Google Scholar
Png, Y. T. et al. Blockade of CD7 expression in T cells for effective chimeric antigen receptor targeting of T-cell malignancies. Blood Adv. 1, 2348–2360 (2017).
Google Scholar
Hu, Y. et al. Sequential CD7 CAR T-cell therapy and allogeneic HSCT without GVHD prophylaxis. N. Engl. J. Med. 390, 1467–1480 (2024).
Google Scholar
Li, S. et al. Eradication of T-ALL cells by CD7-targeted universal CAR-T cells and initial test of ruxolitinib-based CRS management. Clin. Cancer Res. 27, 1242–1246 (2021).
Google Scholar
Sumida, T. S., Cheru, N. T. & Hafler, D. A. The regulation and differentiation of regulatory T cells and their dysfunction in autoimmune diseases. Nat. Rev. Immunol. 24, 503–517 (2024).
Google Scholar
Dominguez-Villar, M. & Hafler, D. A. Regulatory T cells in autoimmune disease. Nat. Immunol. 19, 665–673 (2018).
Google Scholar
Ho, P. et al. Harnessing regulatory T cells to establish immune tolerance. Sci. Transl. Med. 16, eadm8859 (2024).
Google Scholar
Bader, C. S. et al. Single-center randomized trial of Treg graft alone vs Treg graft plus tacrolimus for the prevention of acute GVHD. Blood Adv. 8, 1105–1115 (2024).
Google Scholar
Brunstein, C. G. et al. Infusion of ex vivo expanded T regulatory cells in adults transplanted with umbilical cord blood: safety profile and detection kinetics. Blood 117, 1061–1070 (2011).
Google Scholar
Rodger, B. et al. Protocol for a first-in-human feasibility study of T regulatory cells (TR004) for inflammatory bowel disease using (ex vivo) Treg expansion (TRIBUTE). BMJ Open 15, e092733 (2025).
Google Scholar
Oo, Y. H. et al. Liver homing of clinical grade Tregs after therapeutic infusion in patients with autoimmune hepatitis. JHEP Rep. 1, 286–296 (2019).
Google Scholar
Bender, C. et al. A phase 2 randomized trial with autologous polyclonal expanded regulatory T cells in children with new-onset type 1 diabetes. Sci. Transl. Med. 16, eadn2404 (2024).
Google Scholar
Brunstein, C. G. et al. Adoptive transfer of umbilical cord blood-derived regulatory T cells and early viral reactivation. Biol. Blood Marrow Transplant. 19, 1271–1273 (2013).
Google Scholar
Zielinski, M. et al. Combined therapy with CD4+ CD25highCD127− T regulatory cells and anti-CD20 antibody in recent-onset type 1 diabetes is superior to monotherapy: randomized phase I/II trial. Diabetes Obes. Metab. 24, 1534–1543 (2022).
Google Scholar
PolTREG. PolTREG Treg cell therapy for patients with type-1 diabetes shows long-term clinical remission and insulin independence https://poltreg.com/poltreg-treg-cell-therapy-for-patients-with-type-1-diabetes-shows-long-term-clinical-remission-and-insulin-independence (2024).
Tenspolde, M. et al. Regulatory T cells engineered with a novel insulin-specific chimeric antigen receptor as a candidate immunotherapy for type 1 diabetes. J. Autoimmun. 103, 102289 (2019).
Google Scholar
Blat, D., Zigmond, E., Alteber, Z., Waks, T. & Eshhar, Z. Suppression of murine colitis and its associated cancer by carcinoembryonic antigen-specific regulatory T cells. Mol. Ther. 22, 1018–1028 (2014).
Google Scholar
De Paula Pohl, A. et al. Engineered regulatory T cells expressing myelin-specific chimeric antigen receptors suppress EAE progression. Cell. Immunol. 358, 104222 (2020).
Google Scholar
Fransson, M. et al. CAR/FoxP3-engineered T regulatory cells target the CNS and suppress EAE upon intranasal delivery. J. Neuroinflammation 9, 112 (2012).
Google Scholar
Raffin, C. et al. Development of citrullinated-vimentin-specific CAR for targeting Tregs to treat autoimmune rheumatoid arthritis. J. Immunol. 200, 176.117 (2018).
Kohler, M. et al. A phase 1 study of autologous CAR-Treg cells in refractory rheumatoid arthritis: interim report of safety and efficacy. Arthritis Rheumatol. 77, LB23 (2025).
Sagoo, P. et al. Human regulatory T cells with alloantigen specificity are more potent inhibitors of alloimmune skin graft damage than polyclonal regulatory T cells. Sci. Transl. Med. 3, 83ra42 (2011).
Google Scholar
Smith, B. M., Lyle, M. J., Chen, A. C. & Miao, C. H. Antigen-specific in vitro expansion of factor VIII-specific regulatory T cells induces tolerance in hemophilia A mice. J. Thromb. Haemost. 18, 328–340 (2020).
Google Scholar
Uenishi, G. I. et al. GNTI-122: an autologous antigen-specific engineered Treg cell therapy for type 1 diabetes. JCI Insight 9, e171844 (2024).
Google Scholar
Ohkura, N. et al. T cell receptor stimulation-induced epigenetic changes and Foxp3 expression are independent and complementary events required for Treg cell development. Immunity 37, 785–799 (2012).
Google Scholar
Kitagawa, Y. & Sakaguchi, S. Molecular control of regulatory T cell development and function. Curr. Opin. Immunol. 49, 64–70 (2017).
Google Scholar
Mikami, N. et al. Epigenetic conversion of conventional T cells into regulatory T cells by CD28 signal deprivation. Proc. Natl. Acad. Sci. USA 117, 12258–12268 (2020).
Google Scholar
Akamatsu, M. et al. Conversion of antigen-specific effector/memory T cells into Foxp3-expressing Treg cells by inhibition of CDK8/19. Sci. Immunol. 4, eaaw2707 (2019).
Chen, K. Y. et al. Genome-wide CRISPR screen in human T cells reveals regulators of FOXP3. Nature 642, 191–200 (2025). This study uncovers a new epigenetic checkpoint controlling FOXP3 induction, advancing the mechanistic foundation for generating stable, clinically applicable iTreg cells in autoimmune and inflammatory diseases.
Google Scholar
Mukai, M. et al. Conversion of pathogenic T cells into functionally stabilized Treg cells for antigen-specific immunosuppression in pemphigus vulgaris. Sci. Transl. Med. 17, adq9913 (2025).
Google Scholar
Mikami, N. et al. Generation of antigen-specific and functionally stable Treg cells from effector/memory T cells for cell therapy of immunological diseases. Sci. Transl. Med. 17, adr6049 (2025).
Google Scholar
Simonetta, F., Alvarez, M. & Negrin, R. S. Natural killer cells in graft-versus-host-disease after allogeneic hematopoietic cell transplantation. Front. Immunol. 8, 465 (2017).
Google Scholar
Cichocki, F., van der Stegen, S. J. C. & Miller, J. S. Engineered and banked iPSCs for advanced NK- and T-cell immunotherapies. Blood 141, 846–855 (2023).
Google Scholar
Myers, J. A. & Miller, J. S. Exploring the NK cell platform for cancer immunotherapy. Nat. Rev. Clin. Oncol. 18, 85–100 (2021).
Google Scholar
Herrera, L. et al. The race of CAR therapies: CAR-NK cells for fighting B-cell hematological cancers. Cancers 13, 5418 (2021).
Google Scholar
Moscarelli, J., Zahavi, D., Maynard, R. & Weiner, L. M. The next generation of cellular immunotherapy: chimeric antigen receptor-natural killer cells. Transplant. Cell. Ther. 28, 650–656 (2022).
Google Scholar
Gregoire, C. et al. The trafficking of natural killer cells. Immunol. Rev. 220, 169–182 (2007).
Google Scholar
Rapp, M., Wiedemann, G. M. & Sun, J. C. Memory responses of innate lymphocytes and parallels with T cells. Semin. Immunopathol. 40, 343–355 (2018).
Google Scholar
Marin, D. et al. Safety, efficacy and determinants of response of allogeneic CD19-specific CAR-NK cells in CD19+ B cell tumors: a phase 1/2 trial. Nat. Med. 30, 772–784 (2024).
Google Scholar
Jorgensen, L. V., Christensen, E. B., Barnkob, M. B. & Barington, T. The clinical landscape of CAR NK cells. Exp. Hematol. Oncol. 14, 46 (2025).
Google Scholar
Gao, J. et al. Allogenic CD19 CAR NK cell therapy in refractory systemic lupus erythematosus—a case series study. Ann. Rheum. Dis. 84, 321 (2025).
Google Scholar
Hayday, A., Dechanet-Merville, J., Rossjohn, J. & Silva-Santos, B. Cancer immunotherapy by γδ T cells. Science 386, eabq7248 (2024).
Google Scholar
Bertaina, A. et al. HLA-haploidentical stem cell transplantation after removal of αβ+ T and B cells in children with nonmalignant disorders. Blood 124, 822–826 (2014).
Google Scholar
Mensurado, S., Blanco-Dominguez, R. & Silva-Santos, B. The emerging roles of γδ T cells in cancer immunotherapy. Nat. Rev. Clin. Oncol. 20, 178–191 (2023).
Google Scholar
Yazdanifar, M., Barbarito, G., Bertaina, A. & Airoldi, I. γδ T cells: the ideal tool for cancer immunotherapy. Cells 9, 1305 (2020).
Google Scholar
Wilhelm, M. et al. Successful adoptive transfer and in vivo expansion of haploidentical γδ T cells. J. Transl. Med. 12, 45 (2014).
Google Scholar
Neelapu, S. S. et al. A phase 1 study of ADI-001: anti-CD20 CAR-engineered allogeneic gamma delta (γδ) T cells in adults with B-cell malignancies. J. Clin. Oncol. 40, 7509 (2022).
Google Scholar
Adicet Bio. Adicet Bio announces positive preliminary data from ADI-001 phase 1 study in patients with lupus nephritis (LN) and systemic lupus erythematosus (SLE) https://investor.adicetbio.com/news-releases/news-release-details/adicet-bio-announces-positive-preliminary-data-adi-001-phase-1 (2025).
Klebanoff, C. A., Khong, H. T., Antony, P. A., Palmer, D. C. & Restifo, N. P. Sinks, suppressors and antigen presenters: how lymphodepletion enhances T cell-mediated tumor immunotherapy. Trends Immunol. 26, 111–117 (2005).
Google Scholar
Wang, L. X., Shu, S. & Plautz, G. E. Host lymphodepletion augments T cell adoptive immunotherapy through enhanced intratumoral proliferation of effector cells. Cancer Res. 65, 9547–9554 (2005).
Google Scholar
Cohen, A. D. et al. B cell maturation antigen-specific CAR T cells are clinically active in multiple myeloma. J. Clin. Invest. 129, 2210–2221 (2019).
Google Scholar
Illei, G. G. et al. Long-term effects of combination treatment with fludarabine and low-dose pulse cyclophosphamide in patients with lupus nephritis. Rheumatology 46, 952–956 (2007).
Google Scholar
Carmona-Rivera, C. & Kaplan, M. J. Low-density granulocytes in systemic autoimmunity and autoinflammation. Immunol. Rev. 314, 313–325 (2023).
Google Scholar
Bar-Or, A. & Li, R. Cellular immunology of relapsing multiple sclerosis: interactions, checks, and balances. Lancet Neurol. 20, 470–483 (2021).
Google Scholar
Sockolosky, J. T. et al. Selective targeting of engineered T cells using orthogonal IL-2 cytokine–receptor complexes. Science 359, 1037–1042 (2018).
Google Scholar
He, J. Z. et al. A consideration of fixed dosing versus body size-based dosing strategies for chimeric antigen receptor T-cell therapies. Clin. Pharmacol. Drug Dev. 11, 1130–1135 (2022).
Google Scholar
Ghassemi, S. et al. Reducing ex vivo culture improves the antileukemic activity of chimeric antigen receptor (CAR) T cells. Cancer Immunol. Res. 6, 1100–1109 (2018).
Google Scholar
Wobma, H. et al. CAR T cell therapy for children with rheumatic disease: the time is now. Nat. Rev. Rheumatol. 21, 494–506 (2025).
Google Scholar
Krickau, T. et al. CAR T-cell therapy rescues adolescent with rapidly progressive lupus nephritis from haemodialysis. Lancet 403, 1627–1630 (2024).
Google Scholar
Malvar, A. et al. Histologic versus clinical remission in proliferative lupus nephritis. Nephrol. Dial. Transplant. 32, 1338–1344 (2017).
Google Scholar
Joly, P. et al. First-line rituximab combined with short-term prednisone versus prednisone alone for the treatment of pemphigus (Ritux 3): a prospective, multicentre, parallel-group, open-label randomised trial. Lancet 389, 2031–2040 (2017).
Google Scholar
Cheng, S. W. et al. Monitoring disease activity in pemphigus with enzyme-linked immunosorbent assay using recombinant desmogleins 1 and 3. Br. J. Dermatol. 147, 261–265 (2002).
Google Scholar
Aguirre, F. et al. C3, C5a and anti-acetylcholine receptor antibody as severity biomarkers in myasthenia gravis. Ther. Adv. Neurol. Disord. 13, 1756286420935697 (2020).
Google Scholar
Luo, L. et al. Exploring the clinical significance of anti-acetylcholine receptor antibody titers, changes, and change rates in myasthenia gravis. Front. Neurol. 15, 1506845 (2024).
Google Scholar
Nowak, R. J. et al. Phase 2 trial of rituximab in acetylcholine receptor antibody-positive generalized myasthenia gravis: the BeatMG study. Neurology 98, e376–e389 (2022).
Google Scholar
Lopez-Hoyos, M. et al. Clinical disease activity and titers of anti-dsDNA antibodies measured by an automated immunofluorescence assay in patients with systemic lupus erythematosus. Lupus 14, 505–509 (2005).
Google Scholar
Lazarus, M. N., Turner-Stokes, T., Chavele, K. M., Isenberg, D. A. & Ehrenstein, M. R. B-cell numbers and phenotype at clinical relapse following rituximab therapy differ in SLE patients according to anti-dsDNA antibody levels. Rheumatology 51, 1208–1215 (2012).
Google Scholar
Junt, T. et al. Defining immune reset: achieving sustained remission in autoimmune diseases. Nat. Rev. Immunol. 25, 528–541 (2025).
Google Scholar
Jiang, R. et al. Single-cell repertoire tracing identifies rituximab-resistant B cells during myasthenia gravis relapses. JCI Insight 5, e136471 (2020).
Google Scholar
Sato, Y., Silina, K., van den Broek, M., Hirahara, K. & Yanagita, M. The roles of tertiary lymphoid structures in chronic diseases. Nat. Rev. Nephrol. 19, 525–537 (2023).
Google Scholar
Zhou, S. et al. Autoreactive B cell differentiation in diffuse ectopic lymphoid-like structures of inflamed pemphigus lesions. J. Invest. Dermatol. 140, 309–318 (2020).
Google Scholar
Wardemann, H. et al. Predominant autoantibody production by early human B cell precursors. Science 301, 1374–1377 (2003).
Google Scholar
Tur, C. et al. CD19-CAR T-cell therapy induces deep tissue depletion of B cells. Ann. Rheum. Dis. 84, 106–114 (2025).
Google Scholar
Welte, T. et al. Identification of covariates modulating B-cell repopulation kinetics in subjects receiving rituximab treatment. Arthritis Rheumatol. 75, 2045–2053 (2023).
Google Scholar
Colliou, N. et al. Long-term remissions of severe pemphigus after rituximab therapy are associated with prolonged failure of desmoglein B cell response. Sci. Transl. Med. 5, 175ra130 (2013).
Google Scholar
Hagen, M. et al. BCMA-targeted T-cell-engager therapy for autoimmune disease. N. Engl. J. Med. 391, 867–869 (2024).
Google Scholar
Wang, Q. et al. In vivo CD19 CAR T-cell therapy for refractory systemic lupus erythematosus. N. Engl. J. Med. 393, 1542–1544 (2025).
Google Scholar
Schett, G. et al. Updated phase 1 trial data assessing the tolerability, efficacy, pharmacokinetics, and pharmacodynamics of BMS-986353 (CC-97540), a CD19-directed chimeric antigen receptor T cell therapy using a next-generation process for severe refractory systemic lupus erythematosus https://www.congressconnection.com/assets/cdx001/acr-2025/ACR2025_Schett_.pdf (2025).
Bristol Myers Squibb. Bristol Myers Squibb presents encouraging data from phase 1 breakfree-1 study of CD19 NEX-T CAR T cell therapy in three chronic autoimmune diseases at ACR Convergence 2025 https://news.bms.com/news/corporate-financial/2025/Bristol-Myers-Squibb-Presents-Encouraging-Data-from-Phase-1-Breakfree-1-Study-of-CD19-NEX-T-CAR-T-Cell-Therapy-in-Three-Chronic-Autoimmune-Diseases-at-ACR-Convergence-2025/default.aspx (2025).
Aggarwal, R. et al. Promising early outcomes with BMS-986353, a CD19-directed chimeric antigen receptor T cell therapy in severe refractory idiopathic inflammatory myopathies: safety and efficacy findings from the ongoing phase 1 trial https://www.congressconnection.com/assets/cdx001/acr-2025/ACR2025_Breakfree-1_IIM_LB+poster_.pdf (2025).
Sheikh, S. et al. RESET-SLE: clinical trial evaluating Rese-cel (resecabtagene autoleucel), a fully human, autologous 4-1BB CD19-CAR T cell therapy in non-renal SLE and lupus nephritis. Arthritis Rheumatol. 77, 2468 (2025).
Khanna, D. et al. RESET-SSc: clinical trial evaluating Rese-cel (resecabtagene autoleucel), a fully human, autologous 4-1BB CD19-CAR T cell therapy in systemic sclerosis. Arthritis Rheumatol. 77, 1563 (2025).
Wilfong, E. et al. RESET-Myositis: clinical trial evaluating Rese-cel (resecabtagene autoleucel), a fully human, autologous 4-1BB CD19-CAR T cell therapy in idiopathic inflammatory myopathies. Arthritis Rheumatol. 77, 2669 (2025).
Hagen, M. et al. Safety and efficacy of autologous CD19-CAR T-cell therapy in patients with autoimmune disease—data from the CASTLE phase I/II basket study. Arthritis Rheumatol. 77, 0641 (2025).
Cortés-Hernández, J. et al. Preliminary results of an open-label, multicentre, phase 1/2 study to assess safety, efficacy, and cellular kinetics of YTB323 (rapcabtagene autoleucel), a rapidly manufactured CAR T-cell therapy targeting CD19 on B cells, for severe refractory systemic lupus erythematosus. Ann. Rheum. Dis. 83, 327–328 (2024).
Google Scholar
Zhao, J. et al. Anti-CD19 chimeric antigen receptor T cell therapy for refractory systemic lupus erythematosus: an open-label pilot study. Arthritis Rheumatol. 77, 0647 (2025).
Leandro, M. et al. Obecabtagene autoleucel (obe-cel), a CD19-targeting autologous chimeric antigen receptor T-cell therapy (CAR T) with a fast off-rate binding domain, in patients (pts) with severe, refractory systemic lupus erythematosus (srSLE): preliminary results from the phase I CARLYSLE study. Arthritis Rheumatol. 77, 2458 (2025).
Parker, K. R. et al. Single-cell analyses identify brain mural cells expressing CD19 as potential off-tumor targets for CAR-T immunotherapies. Cell 183, 126–142 (2020).
Google Scholar
Van Oekelen, O. et al. Neurocognitive and hypokinetic movement disorder with features of parkinsonism after BCMA-targeting CAR-T cell therapy. Nat. Med. 27, 2099–2103 (2021).
Google Scholar
Marella, M. et al. Comprehensive BCMA expression profiling in adult normal human brain suggests a low risk of on-target neurotoxicity in BCMA-targeting multiple myeloma therapy. J. Histochem. Cytochem. 70, 273–287 (2022).
Google Scholar